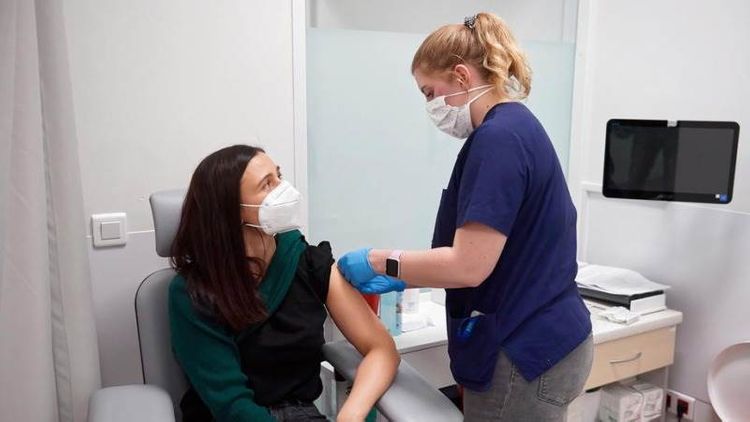
Pfizer Inc and its partner BioNTech Se announced that there is a possibility for "participants who learn they received the placebo, to have the option to receive the investigational vaccine while staying in the study," APA reports citing Teletrader.
Participants who received the placebo will, therefore, get two doses of the investigational vaccine reserved for them within the study and, according to the announcement, "the study doctor will follow the latest guidance from the US Centers for Disease Control and Prevention and their local health authorities to offer the Vaccine Transition Option to participants in a prioritized manner."
The investigational vaccine is allowed to be used during the current COVID-19 public health emergency. The vaccine has not been approved or licensed by the FDA but "has been authorized for emergency use by the FDA under a EUA to prevent COVID-19 for use in individuals 16 years of age and older."